Acoustic Tweezing Cytometry

Mechanosensitivity to extracellular mechanical signals is central to many developmental, physiological, and pathological processes, affecting cell functions including growth, migration, differentiation, and apoptosis. Understanding the molecular mechanisms underlying the mechanotransduction process rely on tools capable of applying controlled mechanical forces to cells to elicit and assess cellular responses.
A novel ultrasound-based technology, acoustic tweezing cytometry (ATC), has been developed as a cell mechanics and mechanobiology tool. The premise of ATC is to bind microbubbles to the cells at controlled points, such as focal adhesions through integrin binding. Then, ultrasound is applied and the bubbles translate this cyclic force to the cell through movement and fluid microstreaming. In this research, we will develop and demonstrate the utility of ATC for stem cell applications, specifically to enable novel advances in human pluripotent stem cell (hPSC) maintenance/differentiation and understanding of mechanobiology of hPSCs. Capable of replicating themselves while retaining the ability to give rise to any type of specialized cells, hPSCs provide promising sources for disease modeling, drug screenings, and future cell-based therapeutics to treat degenerative diseases such as diabetes mellitus and spinal cord injury. Using bubble functionalization, the microbubbles can be bound to many different targets thus allowing a wide selection of force levers to use in studying mechanobiology.
ATC provides advantages over other mechanical manipulation of cells. It is not limited to single-cell analysis, it does not require expensive instrumentation, and it is easy to remove the bubbles without leaving behind any exogenous materials. Control of the broadly applied ultrasound field parameters defines the force applied to a large number of cells simultaneously. This technique is under further development to improve the instrumentation and ease of use.
Displacement of one microbubble attached to an NIH 3T3 fibroblast during single bubble ATC. Chen 2015 Biophysical Journal
Displacement of two microbubbles attached to an NIH 3T3 fibroblast during tandem bubble ATC. Chen 2015 Biophysical Journal
Publications
- Topal T, Fan Z, Deng L, Krebsbach PH, Deng CX. Integrin-targeted Cyclic Forces Accelerate Neural Tube-like Rosette Formation from Human Embryonic Stem Cells. Advanced Biosystems. 2019, 1900064. DOI: 10.1002/adbi.201900064
- Hong X, Rzeczycki PM, Keswani RK, Murashov MD, Fan Z, Deng CX, Rosania GR. Acoustic tweezing cytometry for mechanical phenotyping of macrophages and mechanopharmaceutical cytotripsy. Scientific Reports. 2019. Apr 5;9(1):5702. DOI: 10.1038/s41598-019-42180-3. PMID: 30952950; PMCID: PMC6450871.
- Fan Z, Xu X, Perera R, Esfahani SN, Exner AA, Fu J, Deng CX. Acoustic actuation of integrin-bound microbubbles for mechanical phenotyping during differentiation and morphogenesis of human embryonic stem cells. Small. 2018. 14(50):1803137. https://doi.org/10.1002/smll.201803137. PMID: 30427572
- Xue X, Hong X, Li Z, Deng CX, Fu J. Acoustic tweezing cytometry enhances osteogenesis of human mesenchymal stem cells through cytoskeletal contractility and YAP activation. Biomaterials. 2017; 134:22-30. PMID: 28453955; PMCID: PMC5506541
- Chen D, Sun Y, Gudur MSR, Hsiao Y, Wu Z, Fu J, Deng CX. Two bubble acoustic tweezing cytometry for biomechanical probing and stimulation of cells. Biophysical Journal. 2015;108(1):32-42. PMCID: PMC4286600
- Fan Z, Sun Y, Chen D, Tay D, Chen W, Deng CX, Fu J. Acoustic tweezing cytometry for live-cell subcellular modulation of intracellular cytoskeleton contractility. Scientific Reports. 2013 Jul 12;3:2176.
Resonant Acoustic Rheometry

Resonant acoustic rheometry (RAR) is a recently developed non-contact technique using ultrasound to characterize soft and viscoelastic materials. Ultrasound from a single pushing transducer is precisely targeted at the surface of a sample to create a microscale perturbation, which is measured using an imaging transducer. The resulting displacement dynamics and resonant frequencies, together with Rayleigh and capillary wave modelling, translate to storage and loss modulus and damping coefficient values. This novel measurement of material properties 1) can be applied to virtually any soft, homogenous sample within a well plate, which avoids the need for a rheometer or its clean-up, 2) is easily applied across time for longitudinal studies, and 3) requires only a small sample volume. There is no risk of contamination or damage of the material being tested.
RAR has been applied to various hydrogels and is currently used to study thrombosis. RAR holds great promise as a high-throughput evaluation for biomaterials. The instrumentation is under further development to increase efficiency allowing improved time resolution.
Publications
- Hobson EC, Li W, Juliar BA, Putnam AJ, Stegemann JP, Deng CX. Resonant acoustic rheometry for non-contact characterization of viscoelastic biomaterials. Biomaterials. Feb 2021. 10.1016/j.biomaterials.2021.120676
- Hong X, Annamalai RT, Kemerer T, Deng CX, Stegemann JP. Multimode Ultrasound Viscoelastography for Three-Dimensional Interrogation of Microscale Mechanical Properties in Heterogeneous Biomaterials. Biomaterials. 2018; 178:11-22. doi: 10.1016/j.biomaterials.2018.05.057. PMID 29902533
Sonoporation
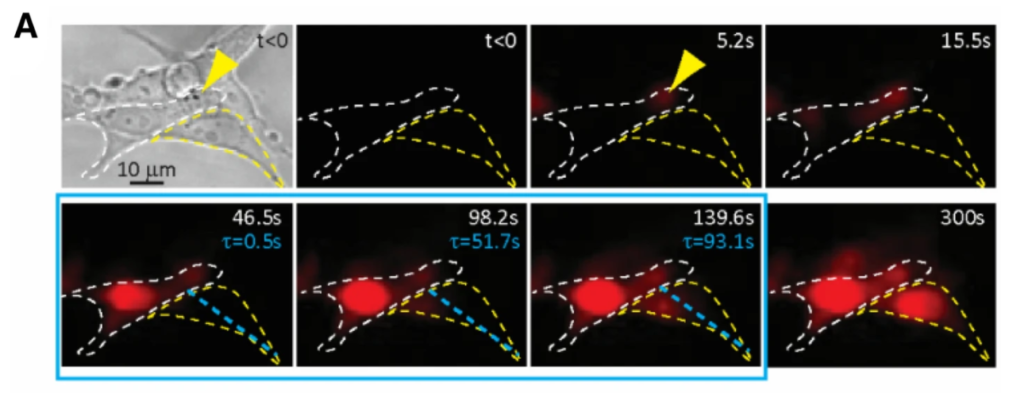
There exists a widely recognized need to develop methods to achieve targeted delivery of drugs and to improve the methods of gene delivery for gene-based therapy of numerous diseases. As ultrasound exposure is safe and non-invasive, and allows targeted application both temporarily and spatially, ultrasound mediated delivery has the potential to provide an advantageous strategy especially for in vivo clinical applications to overcome the limitations of safety concerns, possible mutagenesis and immune responses associated with methods such as electroporation and viral transfection.
It has been demonstrated that ultrasound application results in enhanced intracellular uptake of chemotherapeutic compounds, genetic materials, nanoparticles, and fluorescent dextran molecules, which are normally not permeable through intact cell membranes. The hypothesis is that sonoporation, during which pores form in the cell membrane as the result of ultrasound exposure, allows entry of extracellular molecules and substances into the cell before resealing. However, despite the recent progress made in the field, the mechanisms of sonoporation are not completely understood, and many problems and challenges remain to improve delivery efficiency and cell survival rate.
The long term goal of this research is to develop a robust and reliable ultrasound strategy for intracellular delivery of desirable agents (e.g. drugs, genes, imaging markers) for biomedical applications such as targeted cancer treatment, molecular imaging, and gene therapy. In particular, we focused on investigating the mechanisms of sonoporation by studying the dynamic processes of sonoporation at both the single cell level and the cellular level based on a large number of statistical events.
Publications
- Fan Z, Xue X, Fu J, Deng CX. Visualization and quantification of dynamic intercellular coupling in human embryonic stem cells using single cell sonoporation. Scientific Reports. Oct 2020. 10.1038/s41598-020-75347-4
- Fan Z, Kumon RE, Deng CX. Mechanisms of microbubble-facilitated sonoporation for drug and gene delivery. Therapeutic delivery. 2014; 5(4): 467-486.
- Fan Z, Chen D, Deng CX. Dynamic activities of a population of microbubbles driven by pulsed ultrasound exposures in sonoporation. Ultrasound Med Biol. 2014; 40(6):1260-72.
- Frampton JP, Fan Z, Simon A, Chen D, Deng CX, Takayama S. Aqueous two-phase system patterning of microbubbles for localized sonoporation of cells. Advanced Functional Materials. 2013 Jul 23;27:3366.
- Fan Z, Liu H, Mayer M, Deng CX. Spatiotemporally controlled single cell sonoporation. Proc Natl Acad Sci U S A. 2012 Oct 9;109(41):16486-91.
- Park J, Fan Z, Deng CX. Effects of shear stress cultivation on cell membrane disruption and intracellular calcium concentration in sonoporation of endothelial cells. Journal of Biomechanics 2011. 44:164-169.
- Fan Z, Kumon RE, Park J, Deng CX. Intracellular delivery and calcium transients generated in sonoporation facilitated by microbubbles. Journal of Controlled Release 2010; 142:31-39.
- Kumon RE, Aehle M, Sabens D, Parikh P, Han YW, Kourennyi D, Deng CX. Spatiotemporal effects of sonoporation on mammalian cells measured by real-time calcium imaging. Ultrasound in Med and Biol 2009; 35: 494-506.
Cell Patterning
Soon to be updated
Spectral Ultrasound Imaging

Spectral Ultrasound Imaging (SUSI) is a technique that uses the backscattered radiofrequency spectrum to derive information about the composition of a sample. A key feature of ultrasound techniques is that they are noninvasive and therefore can be used to study developing tissues over time. In addition, imaging and deformation can be applied at sub-millimeter resolution. Importantly, SUSI allows for quantification of ultrasound imaging data. This allows comparison between systems, whereas standard B-mode imaging only allows qualitative measurement of relative hypoechogenicity or hyperechogenicity.
This technique has been applied to studying the microscale physical properties of engineered musculoskeletal tissues composed of cell-seeded mineralizing hydrogels. Mechanical forces are a key component of the cellular microenvironment, and are well established to have potent effects on cells and tissues. The passive mechanical properties of two-dimensional cell substrates and three-dimensional extracellular matrices have been shown to influence progenitor cell phenotype and can be used to direct cell function. Mechanobiology is particularly relevant to musculoskeletal tissues, but there is a gap in our understanding of the physical properties of the tissue environment on length scales that cells sense.
Publications
- Edwards NJ, Hobson E, Dey D, Rhodes A, Overmann A, Hoyt B, Walsh SA, Pagani CA, Strong AL, Hespe GE, Padmanabhan KR, Huber A, Deng CX, Davis TA, Levi B. High Frequency Spectral Ultrasound Imaging Detects Early Heterotopic Ossification in Rodents. Stem Cells and Development. May 2021. https://doi.org/10.1089/scd.2021.0011
- Bushnell GG, Hong X, Hartfield RM, Zhang Y, Oakes RS, Rao SS, Jeruss JS, Stegemann JP, Deng CX, Shea LD. High frequency spectral ultrasound imaging to detect metastasis in implanted biomaterial scaffolds. Ann Biomed Eng. 2019 Sep 23. doi: 10.1007/s10439-019-02366-2.
- Ranganathan K, Hong X, Cholok D, Habbouche J, Priest C, Breuler C, Chung M, Li J, Kaura A, Hsieh HHS, Butts J, Ucer S, Schwartz E, Buchman SR, Stegemann JP, Deng CX, Levi B. High-frequency spectral ultrasound imaging (SUSI) visualizes early post-traumatic heterotopic ossification (HO) in a mouse model. Bone. 2018; 109:49-55. doi: 10.1016/j.bone.2018.01.034. PMID: 29412179
- Gudur MSR, Rao RR, Hsiao Y, Peterson AW, Stegemann JP, Deng CX. Noninvasive Quantification of In Vitro Osteoblastic Differentiation in 3D Engineered Tissue Constructs using Spectral Ultrasound Imaging. Plos One 2014; Jan 22;9(1):e85749. doi: 10.1371/journal.pone.0085749.
